2018年11月
Nanobubbles:The Future
Nanobubbles are less than 1 µm in diameter. Due to its extraordinary chemical and physical characteristics, they have the potential to revolutionize the way of delivering drugs.
Ultra-fine bubbles (nanobubbles) are so small that they cannot be visualized even with the finest optical microscopes. Ultra-fine bubbles are different from ordinary bubbles and are extremely stable. Most bubbles quickly rise to the surface of a liquid and collapse, however, due to the very low buoyancy of ultra-fine bubbles, they will remain suspended in liquids for an extended period of time. Also, the surface area-to-volume ratio per mass is much higher compared to ordinary bubbles. In addition, the bubbles are believed to have negatively charged surface leading to higher capacity of carrying counter ion electrolytes and positively charged materials. Although the nature of ultra-fine bubbles are not fully understood, due to these extraordinary chemical and physical characteristics, they are currently used in the field of agriculture as promoters for plant growth, oyster sterilization and as cleansing agents in industrial and in the food market.

Ultra-fine bubbles (less than 1µmin diameter) visualized with laser scattering.
How are nanobubbles made?
Conventional ultra-fine bubble generators are composed of pressure controlled chambers, high pressure air tanks, special nozzles and sophisticated circulation tubing systems. Liters and liters of liquid are required to generate sufficient amounts of bubbles. Large bubble generators can produce tons of nanobubble included wastewater solutions to eliminated bacteria, algae and fungi in dams and lakes. Such devices are frequently used to produce ultra-fine bubbles in massive volumes.
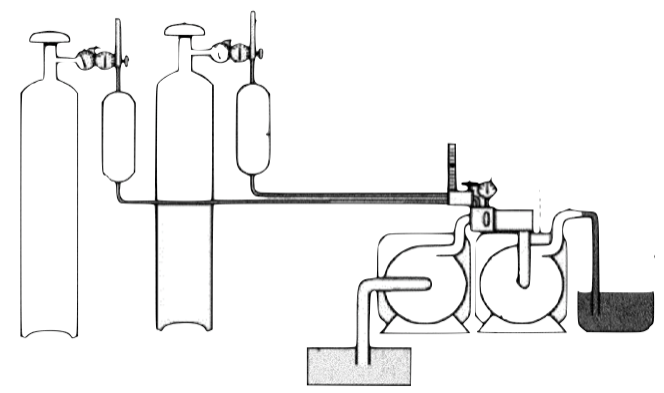
Ultra-fine bubbles. Large air compressors and circulating massive amounts of liquids are necessary to produce bubbles.
Our method of producing bubbles
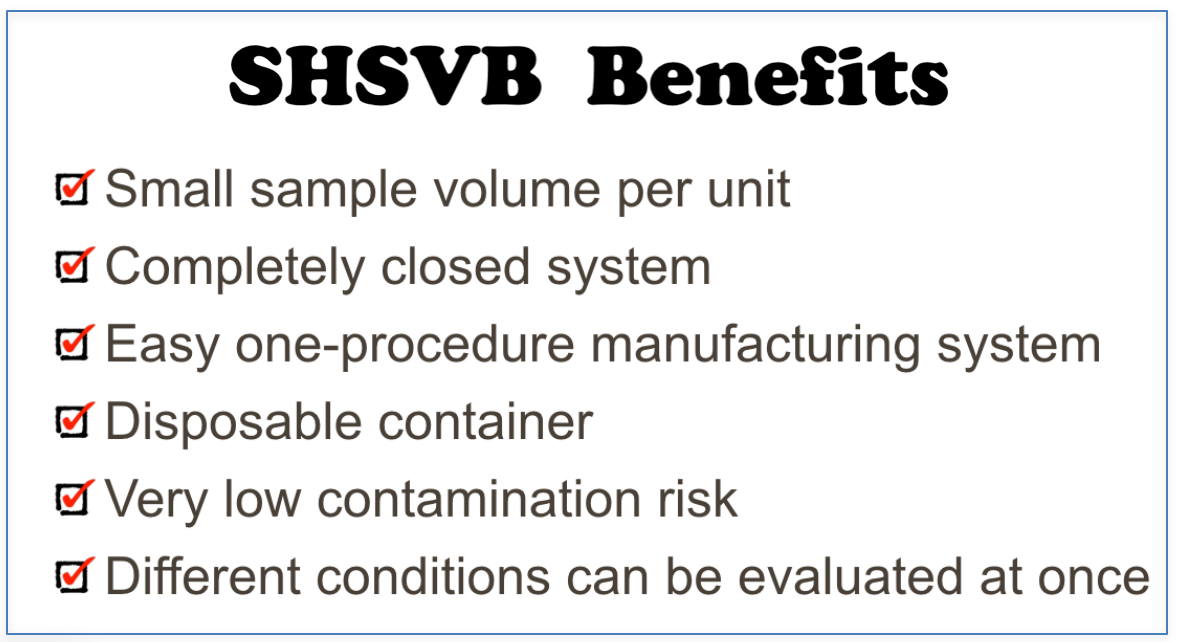
Super High Speed Vibration Bubbling (SHSVB) system is a patented technology developed by scientists at Fukuoka University School of Medicine in Japan. This revolutionary method enables ultra-fine bubbles to be produced in very small volumes (2-15ml) in a closed sterilized environment. The basic mechanism is simple. The container with a specific liquid is pressurized and shaken at super high speeds ranging from 6000 rpm to 10000 rpm. This results in production of highly concentrated and stable ultra-fine bubbles ranging from sizes of 50 to 500 nm. Any gas of choice can be pre-filled in the container to produce for example oxygen, hydrogen, argon loaded nanobubbles. This SHSVB system was first developed under the concept of investigating a possible use of ultra-fine bubbles for the medical field. Conventional bubble generators are unfit for evaluating multi-numbers of experiments with different conditions and materials. In addition, pharmaceutical materials are often expensive and cannot be obtained in massive volume. For example, evaluation of an antibody carrying ultra-fine bubble for oncology treatment purposes is not practical with conventional bubble generating methods and simply out of the question from a financial point of view. Why little research on the medical application of ultra-fine bubble, was in part due to the above reasons.
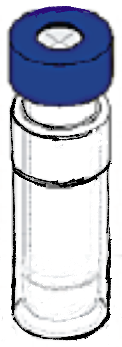
Small air tight pressurized container. The container is shaken at very high velocities horizontally and vertically.

High speed videos show violent turbulent flow within the custom made air tight container.
Our advanced nanobubble measurement technology
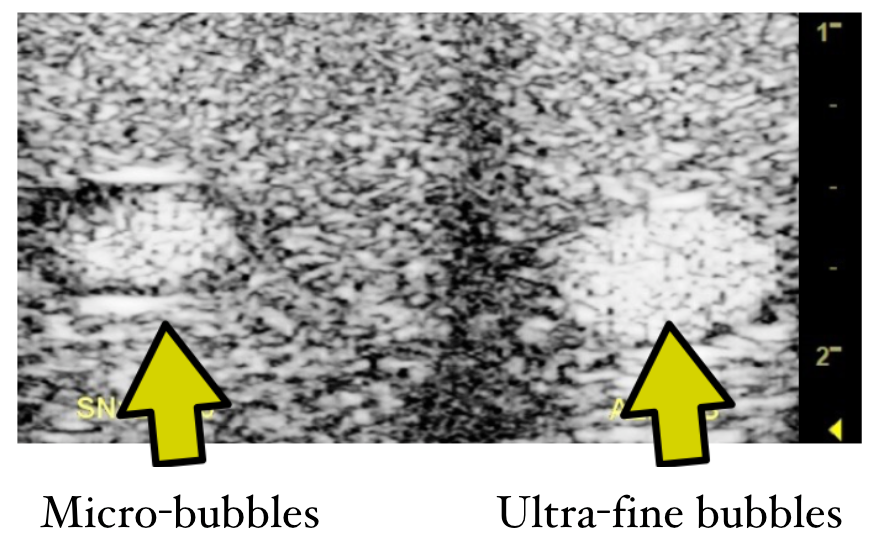
Nano-sized bubbles are very difficult to detect and measure. We have in our lab the most advanced instruments to measure the size distribution, concentration (counting the number of bubbles) and the buoyant mass to distinguish between nano-sized gas/particles. By combining the data obtained from these different measurement modalities, we are now able to estimate the size distribution and characteristics of the bubbles with great accuracy. Our bubble measurement team have created a huge database related to which materials can be “bubblized” to form ultra-fine bubbles as well as the optimal condition, gas type or solution that lead to longer bubble life span. Through our intense research over the years, we discovered the stability of ultra-fine bubbles, together with their high surface area per volume, that can carry specific pharmaceutical materials in the gas-liquid interfaces. Our understanding so far is that unlike micro-bubbles, ultra-fine bubbles do not necessarily need “hard shells” to support their structure thus theoretically, a wide range of soluble drugs can be “bubblized” to some degree to induce beneficial effects within the human body.

NanoSight System which measures and tracks the Brownian movements to calculate the size distribution of ultra-fine bubbles (Malvern Panalytical).

Archimedes is a high-performance system which uses resonant mass measurement in order to detect and count nanoparticle mass and size (Malvern Panalytical).

The CytoFLEX flow cytometer utilizes Avalanche Photodiodes (APDs) with short wave length lasers to detect and count nanobubbles (Beckman Coulter).
Bubbles as an imaging agent
Our experiments have shown ultrasound contrast images of ultra-fine bubbles comparable to conventional micro-bubble contrast products. In some cases, ultra-fine bubbles show higher resolution and different behavior in the vascular system simply due to the smaller size of the bubble. Such basic material as albumin, saline or cell culture medium can be transformed into ultra-fine bubble solutions by our newly developed SHSVB system. These fundamental pharmaceutical agents can be visualized by ultrasound in vitro and in vivo. Drugs can be carried by these bubbles functioning as a drug delivery system modality. Thus, the target pharmaceutical material can be easily visualized and monitored within the body by conventional ultrasound imaging devices. We have discovered that irradiation of relatively higher intensity ultrasound can collapse the bubbles to increase local penetration of drugs into cancer cells. This phenomenon referred as sonoporation can be obtained by combining ultra-fine bubble, a pharmaceutical agent and therapeutic ultrasound. The two stage approach of monitoring the location of ultra-fine bubble and then collapsing them to deliver a drug in a specific location is frequently referred as “theranostic ultrasound”. Ultra-fine bubbles combined with various pharmaceutical products may open up a whole new category of theranostic agents.
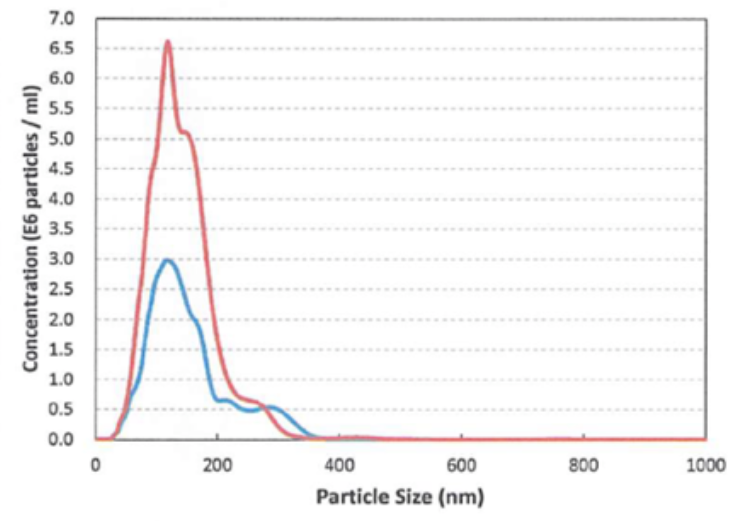
Two different types of Ringer’s solution ultra-fi ne bubbles generated by our SHSVB system.
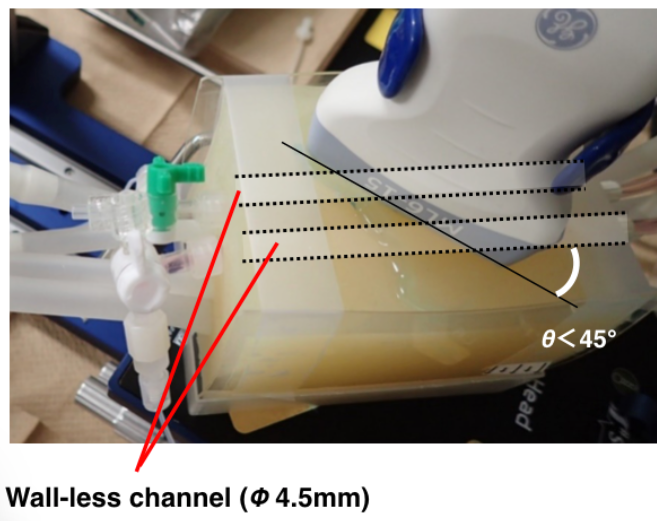
Two channel acoustic phantom studies comparing conventional ultrasound contrast agents (left) and ultra-fine bubbles (right).
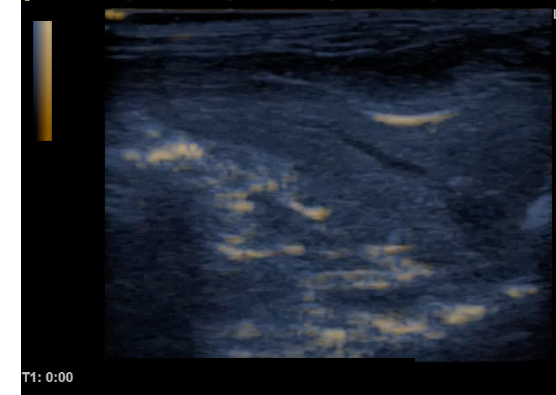
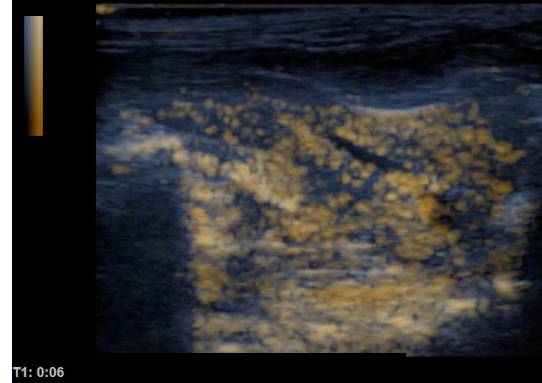
Comparison of ultrasound images of rat liver before (left) and after (right) intra venous injection of albumin based ultra-fine bubbles.
The database
The combination of our ultra-fine bubble generating system and advanced bubble measurement technology enabled us to obtain a wide range of information concerning which materials are easier to “bubblize” and which production protocol should be applied. We have tested hundreds of materials ranging from distilled water to glucose, salt, protein, lipid included solutions. Also, various gas type such as hydrogen, argon, oxygen, helium and others, have each shown different biophysical bubble behavior. Our strength lies on this huge multi-parameter database and know-how obtained through intensive experiments conducted at our lab. Built on this platform, our “core” technology can be apply to futuristic pharmaceutical products or for re-innovating already approved drugs, transforming them into theranostic functioning agents. Because no additional chemical revision is needed in this technology, careful strategic planning could lead to early FDA approval.
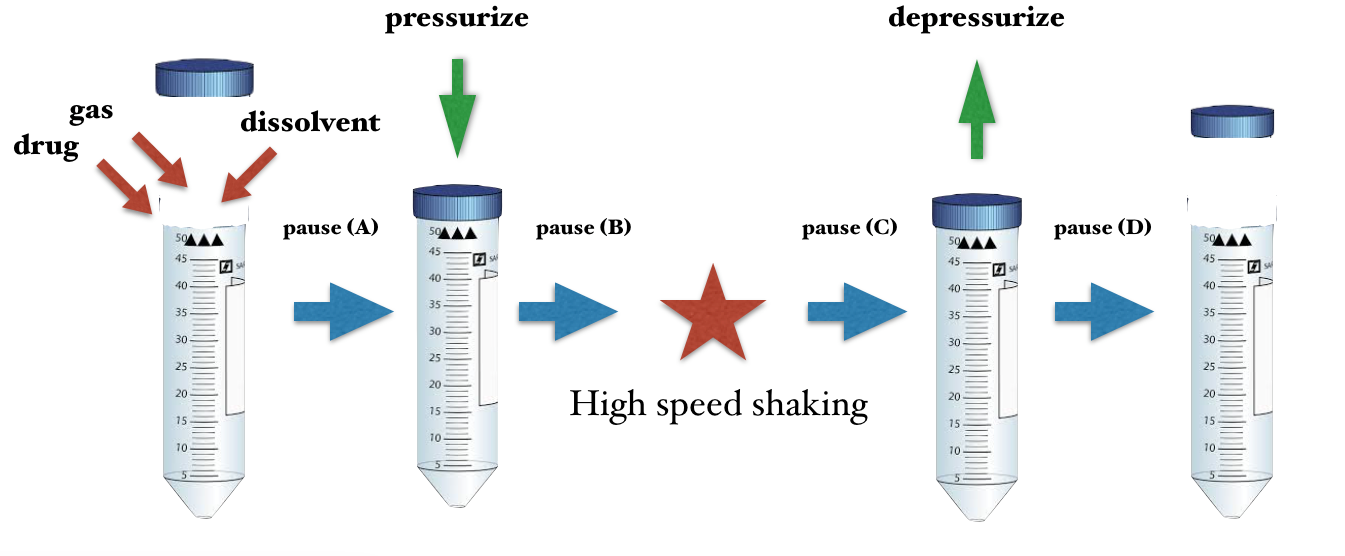
The basic protocol of generating ultra-fine bubbles consists of many conditions, materials and procedures. The combination of these parameters are infinitive but have to some extent, a constant “pattern” that can be directly applied to similar pharmaceutical products.
Intellectual property
In parallel with expanding our database concerning the production of ultra-fine bubble, we have aggressively applied for world wide intellectual property. So far, as of 2018, more than 10 Japanese patents have been applied where 5 have already been granted. More than five PCT patents are in the pending stage.